Physics
Gravitation
Definition
Gravitation is the fundamental natural force of attraction between any two objects with mass. This force is universal, acting on everything from the smallest particles to the largest stars and galaxies throughout the cosmos.
Discovery and Historical Context
While phenomena related to gravity have been observed throughout history, our modern understanding largely stems from these key figures:
- Nicolaus Copernicus and Aryabhata: Proposed heliocentric models, placing the Sun, not the Earth, at the center of our solar system.
- Galileo Galilei: Established that, neglecting air resistance, all objects fall towards the Earth with the same constant acceleration, regardless of their mass.
- Tycho Brahe: Made meticulous observations of planetary positions.
- Johannes Kepler: Using Brahe's data, formulated three laws describing planetary motion.
- Sir Isaac Newton (1687): Synthesized previous work and formulated the Universal Law of Gravitation, providing a mathematical description of the force. The famous anecdote involves him pondering why an apple falls straight down to Earth.
Universal Law of Gravitation
Newton's law states: "Every object in the universe attracts every other object with a force that is directly proportional to the product of their masses and inversely proportional to the square of the distance between their centers."
Mathematically, the force (F) between two objects of masses m₁ and m₂ separated by a distance r is:
Where:
- F is the gravitational force (in Newtons, N)
- m₁ and m₂ are the masses of the two objects (in kilograms, kg)
- r is the distance between the centers of the two objects (in meters, m)
- G is the universal gravitational constant, experimentally determined to be approximately
Characteristics of Gravitational Force
- Attractive: It always pulls objects towards each other; it never pushes them apart.
- Universal: It applies to all objects with mass everywhere in the universe.
- Depends on Mass: The force is stronger for objects with larger masses.
- Depends on Distance: The force decreases rapidly as the distance (r) between objects increases, following an inverse square law (
). - Acts Along a Line: The force acts along the straight line connecting the centers of the two objects.
- Weakest Fundamental Force: While it governs large-scale structures, it's intrinsically much weaker than electromagnetic, strong nuclear, and weak nuclear forces. However, its infinite range and the fact that mass is always positive (leading to attraction only) make it dominant on astronomical scales.
Comparison with Magnetic and Electric Forces
| Aspect | Gravitational | Electrical | Magnetic |
|---|---|---|---|
| Nature of Force | Attractive only | Attractive or repulsive | Attractive or repulsive |
| Source | Mass | Electric charge | Moving charges or magnets |
| Force Carrier (Field) | Gravitational field | Electric field | Magnetic field |
| Relative Strength | Weakest of the three | Much stronger than gravity | Depends on motion and configuration |
| Mathematical Law | Newton’s Law of Gravitation | Coulomb’s Law | Lorentz Force Law |
| Acts on | Masses | Electric charges | Moving charges and magnetic materials |
| Field Lines | Always point toward mass (inward) | From +ve to –ve charge | From N to S pole outside magnet, closed loops |
| Shielding Possible? | No | Yes (via conductors or Faraday cage) | Yes (via soft iron or magnetic shielding) |
| Long Range Force? | Yes | Yes | Yes |
| Affected by Medium? | No | Yes (permittivity of the medium) | Yes (permeability of the medium) |
Acceleration Due to Gravity (g)
When the Earth's gravitational force causes an object to accelerate (like during free fall), this acceleration is called the acceleration due to gravity, denoted by g.
Calculating 'g' on Earth's Surface
Using Newton's Second Law (F = ma) and the Law of Universal Gravitation, where ME is the mass of the Earth, RE is the radius of the Earth, and m is the mass of the falling object:
Equating these gives:
Notice that the mass of the falling object (m) cancels out, confirming Galileo's finding that g is independent of the mass of the falling object (neglecting air resistance).
- The standard average value of g on Earth's surface is approximately 9.8 m/s². This means a freely falling object's downward velocity increases by 9.8 meters per second every second.
Variation in 'g'
The value of g is not perfectly constant; it varies slightly with:
- Altitude (h): g decreases as you move away from the Earth's surface. For heights h much smaller than the Earth's radius (RE), the approximate value is:
- Depth (d): g also decreases as you go deeper below the Earth's surface. Assuming uniform density:
At the center of the Earth (d = RE), g becomes zero. - Latitude and Rotation: Due to the Earth's rotation and its slight bulge at the equator, g is slightly weaker at the equator than at the poles.
'g' on Other Celestial Bodies
The acceleration due to gravity depends on the mass and radius of the planet or moon.
| Planet/Satellite | Gravitational Acceleration (m/s²) | Notes |
|---|---|---|
| Earth | 9.8 | Our reference point |
| Moon | 1.62 | About 1/6th of Earth's 'g' |
| Mars | 3.7 | |
| Jupiter | 24.8 | Strongest in solar system |
| Venus | 8.87 | Similar size/mass to Earth |
Kepler's Laws of Planetary Motion
Johannes Kepler formulated three laws describing how planets orbit the Sun, which were later shown to be consequences of Newton's Law of Universal Gravitation:
- Law of Orbits: Each planet revolves around the Sun in an elliptical orbit, with the Sun located at one focus of the ellipse (not the center). This means a planet's distance from the Sun varies during its orbit, being closest at perihelion and farthest at aphelion.
- Law of Areas: An imaginary line joining a planet and the Sun sweeps out equal areas in equal intervals of time. This implies that a planet moves faster when it is closer to the Sun (near perihelion) and slower when it is farther away (near aphelion). This law is a consequence of the conservation of angular momentum.
- Law of Periods: The square of the orbital period (T) of a planet is directly proportional to the cube of the semi-major axis (a) of its orbit.
For an orbit around a central body of mass M: This means planets farther from the Sun take significantly longer to complete one orbit.
Uses of Kepler's Laws
- Predicting the motion and positions of planets.
- Understanding the structure and dynamics of the solar system.
- Calculating orbits for artificial satellites and space probes.
- Studying exoplanets and other celestial systems.
Escape Velocity (Ve)
Escape velocity is the minimum initial speed an object needs to completely escape the gravitational pull of a celestial body (like a planet or moon) without needing further propulsion. If launched slower than this speed, the object will eventually fall back or enter an orbit.
- Example: For Earth, the escape velocity is approximately 11.2 km/s (about 40,320 km/h or 25,000 mph).
Formula for Escape Velocity
Where:
- Ve is the escape velocity
- G is the universal gravitational constant
- M is the mass of the celestial body (e.g., planet)
- R is the radius of the celestial body
Dependence and Importance
- Escape velocity depends on the mass and radius of the celestial body. Larger mass or smaller radius leads to higher escape velocity.
- Rocket Launches: Spacecraft must reach Earth's escape velocity to travel to other planets or deep space.
- Planetary Atmospheres: Planets with low escape velocity (like Mercury or the Moon) cannot easily hold onto light gas molecules, contributing to their lack of substantial atmospheres. Earth's escape velocity is high enough to retain its atmosphere.
- Black Holes: The gravitational pull is so strong that the escape velocity exceeds the speed of light, meaning nothing, not even light, can escape.
Satellite Motion
A satellite is any object orbiting a larger celestial body due to gravity. Satellites can be natural (like the Moon orbiting Earth) or artificial (man-made spacecraft).
How Satellites Stay in Orbit
A satellite maintains its orbit due to a balance between two factors:
- Gravitational Force: Pulls the satellite towards the central body.
- Inertia/Velocity: The satellite's forward motion (tangential velocity) causes it to continually "miss" the central body as it falls towards it.
The gravitational force provides the necessary centripetal force to keep the satellite moving in a curved path (orbit).
Orbital Velocity (v)
The speed required for a satellite to maintain a circular orbit at a height h above the surface of a planet with radius R and mass M is:
- Note: r = R+h is the distance from the center of the planet to the satellite.
- For a Low Earth Orbit (LEO) around 300 km altitude, the orbital speed is roughly 7.8 km/s.
Time Period (T)
The time it takes for a satellite to complete one full orbit is its period. Using Kepler's Third Law:
- Height Dependence: Satellites in higher orbits have longer periods.
Types of Artificial Satellites
| Feature | Geostationary (GEO) | Polar (LEO) | |||
|---|---|---|---|---|---|
| Orbit | Equatorial plane | Passing over/near Earth's Poles | |||
| Altitude | ~35,786 km | Few hundred to ~1000 km | |||
| Orbital Period | ~24 hours | ~90-120 minutes | |||
| Motion Relative to Earth | Appears Stationary | Moves Relative to Earth | |||
| Coverage | Fixed area of Earth | Entire Earth surface (over time) | |||
| Uses | Communications, Weather Forecasting | Earth Observation, Mapping, Environmental Monitoring, Reconnaissance, ISS | |||
| Examples | INSAT, GSAT series | (Implicitly) ISS |
Gravitational Potential Energy (U)
This is the energy stored in an object due to its position within a gravitational field.
Formulae
Near Earth's Surface: For an object of mass m at a height h above a reference level (where g is approximately constant):
Here, potential energy increases with height. General Case (Universal Law): The potential energy between two masses M and m separated by a distance r (measured from centers) is:
- The energy is defined as zero when the separation r is infinite.
- The potential energy is always negative, becoming more negative (lower energy state) as the objects get closer. Moving objects farther apart increases their gravitational potential energy (makes it less negative).
Conservation of Energy
In the absence of non-conservative forces like air resistance, the total mechanical energy (Kinetic Energy + Potential Energy) of an object moving under gravity is conserved. As an object falls, its potential energy (
Gravity in Daily Life
Gravitation is constantly at work, shaping our experiences in countless ways:
- Keeping Us Grounded: Earth's gravity pulls us and everything else towards its center, allowing us to walk, run, and sit. Without it, we'd float away. This pull gives us our sense of weight.
- Falling Objects: Anything thrown upwards eventually falls back down due to gravity's relentless pull. This is the classic observation that sparked Newton's insights.
- Water Flow and Rainfall: Gravity pulls rain from clouds to the ground and causes rivers to flow downhill towards the sea.
- Celestial Motion: Gravity holds the Moon in orbit around Earth, and the Earth and other planets in orbit around the Sun. It dictates the structure of solar systems, galaxies, and the universe itself.
- Weight vs. Mass: Your mass (amount of matter) is constant, but your weight (the force of gravity on your mass, W = mg) depends on the local acceleration due to gravity (g). You'd weigh less on the Moon because its g is lower, but your mass remains the same.
- Sports: The parabolic trajectory of a thrown basketball or a cricket ball is shaped by the combination of its initial velocity and the constant downward pull of gravity.
- Tides: The gravitational pulls of the Moon and (to a lesser extent) the Sun cause the rise and fall of sea levels (tides).
- Climbing Difficulty: Climbing uphill requires working against gravity, consuming more energy and making it feel strenuous.
- Astronaut Weightlessness: Astronauts in orbit experience apparent weightlessness because they and their spacecraft are constantly in a state of free fall around the Earth. Gravity is still acting strongly on them, but there's no ground or surface to push back and create the sensation of weight.
- Vehicle Motion: Gravity provides the normal force necessary for tire friction, enabling vehicles to accelerate, turn, and brake effectively.
Gravitation and Modern Science
- Einstein's General Theory of Relativity: Provides a more profound description of gravity, not as a force, but as the curvature of spacetime continuum caused by mass and energy. Massive objects warp the spacetime around them, and other objects follow these curves.
- Gravitational Waves: Ripples in spacetime continuum caused by accelerating massive objects (like merging black holes or neutron stars). Their detection in 2015 confirmed a major prediction of General Relativity and opened a new window for observing the universe.
The Human Eye and Vision
The human eye is a vital and intricate sensory organ responsible for vision, located within protective bony sockets called orbits. It functions much like a camera, capturing light and converting it into neural signals interpreted by the brain.
Structure of the Human Eye
The eye is roughly spherical (about 2.3 cm in diameter) and composed of several key parts arranged in layers:
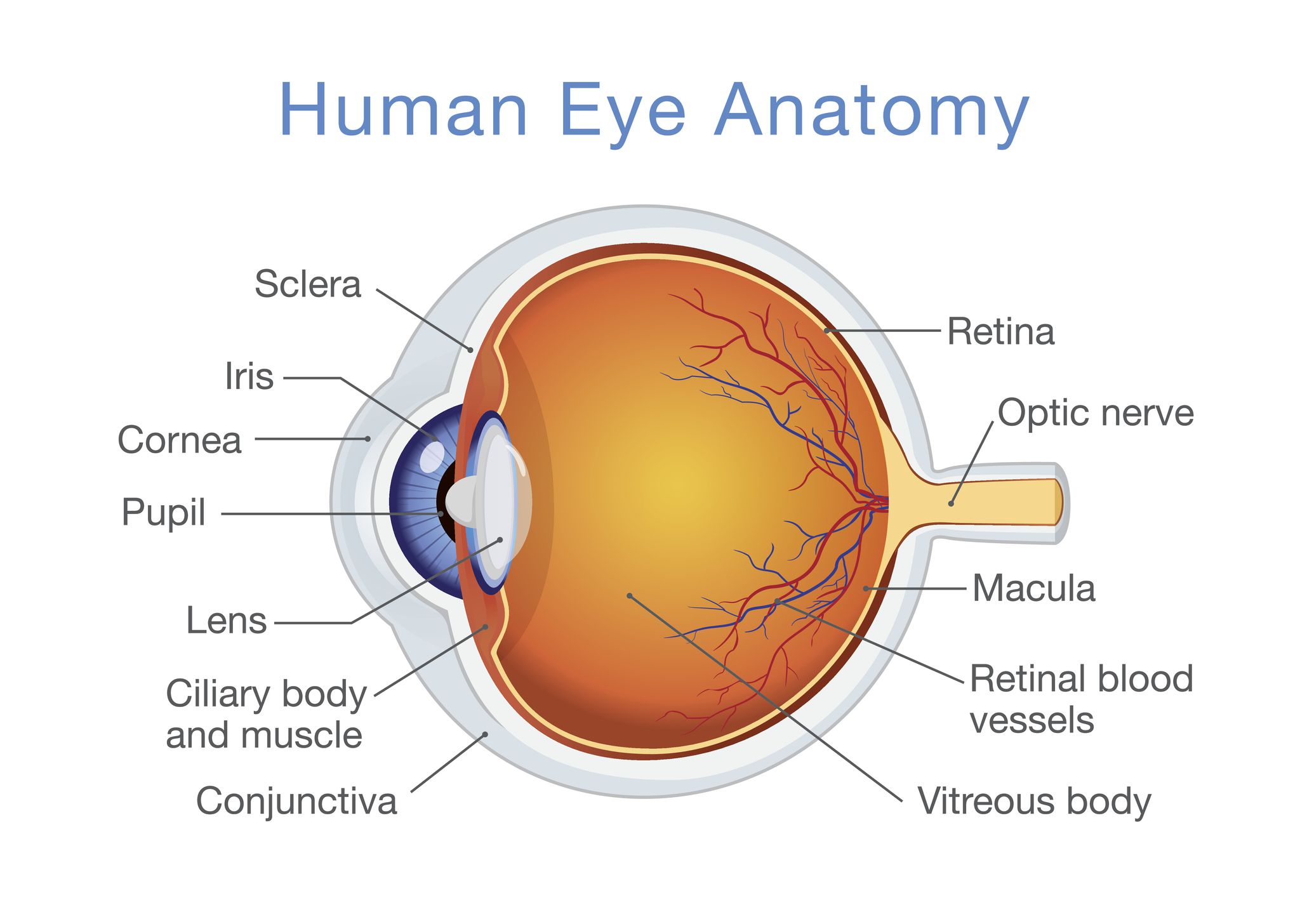
Outer Layer
- Sclera: The tough, white, outer connective tissue providing structural support.
- Cornea: The transparent, curved front part of the sclera. It allows light to enter and performs most of the initial light refraction. It lacks blood vessels, receiving oxygen directly from the air.
Middle Layer (Uvea)
- Choroid: A layer rich in blood vessels, located beneath the sclera, providing nourishment.
- Ciliary Body: An extension of the choroid at the front of the eye, containing the ciliary muscles.
- Ciliary Muscles: Control the shape and thickness of the lens for focusing (accommodation).
- Iris: The colored, muscular diaphragm (e.g., brown, blue, green) extending from the ciliary body. It controls the size of the pupil.
- Pupil: The adjustable opening at the center of the iris. It regulates the amount of light entering the eye – constricting in bright light and dilating in dim light.
Inner Layer
- Retina: The light-sensitive innermost layer lining the back of the eye. It contains photoreceptor cells:
- Rods: Sensitive to dim light (responsible for twilight vision).
- Cones: Sensitive to bright light and responsible for color vision.
- Macula (Yellow Spot): A small area on the retina responsible for sharp, detailed central vision.
- Fovea: A tiny pit in the center of the macula, densely packed with cones, providing the *clearest vision (visual acuity).
- Optic Nerve: A bundle of nerve fibers that transmits visual information (electrical signals) from the retina to the brain.
- Blind Spot: The point where the optic nerve leaves the eye; it lacks photoreceptor cells, creating a gap in the visual field.
Internal Components
- Crystalline Lens: A transparent, biconvex, flexible structure located behind the iris and pupil. It fine-tunes the focus of light onto the retina. Its shape is adjusted by the ciliary muscles.
- Aqueous Humor: A clear, watery fluid filling the aqueous chamber (space between the cornea and the lens). It nourishes the cornea and lens and maintains pressure.
- Vitreous Humor: A clear, jelly-like substance filling the vitreous chamber (the large space behind the lens). It helps maintain the eye's spherical shape and supports the retina.
- Lacrimal Glands: Produce tears to keep the eye moist and protect it from infection.
Mechanism of Vision
Power of Accommodation
Accommodation is the eye's ability to adjust the focal length of its lens to form a clear image on the retina, regardless of the object's distance.
| Object Distance | Ciliary Muscles | Lens Shape | Focal Length |
|---|---|---|---|
| Distant (Infinity) | Relaxed | Thinner (Flatter) | Longer |
| Near | Contracted | Thicker (More Rounded) | Shorter |
Limits of Accommodation:
- Near Point: The closest distance at which an object can be seen clearly without strain. For a normal young adult eye, this is approximately 25 cm.
- Far Point: The farthest distance at which an object can be seen clearly. For a normal eye, this is considered to be infinity.
Vision Defects and Their Correction
When the eye cannot focus light correctly onto the retina, vision becomes blurred. Common defects include:
| Defect | Description | Symptoms | Cause(s) | Correction | Lens Type |
|---|---|---|---|---|---|
| Myopia | Nearsightedness | Distant objects blurry, near objects clear | Eyeball too long; Excessive lens curvature (high power) | Diverging lens | Concave |
| Hypermetropia | Farsightedness | Near objects blurry, distant objects clear | Eyeball too short; Insufficient lens curvature (low power) | Converging lens | Convex |
| Presbyopia | Age-related difficulty focusing on near objects | Near objects blurry | Weakening ciliary muscles; Decreased lens flexibility | Converging / Bifocal lens | Convex |
| Astigmatism | Blurred or distorted vision at all distances | Objects appear distorted, especially edges | Irregular curvature of the cornea or lens | Cylindrical lens | Cylindrical |
| Cataract | Clouding of the lens | Blurry vision, glare, poor night vision | Aging, diabetes, UV exposure (protein buildup in lens) | Surgery (Lens replacement) | N/A |
- Bifocal Lenses: Used often for presbyopia, especially when combined with myopia or hypermetropia. They typically have a concave lens portion (top) for distance and a convex lens portion (bottom) for near vision.
Power of a Lens
The degree of convergence or divergence of light rays achieved by a lens is expressed by its power (
- Formula: Power is the reciprocal of the focal length (
) in meters: - Unit: The SI unit of power is the dioptre (D).
- 1 Dioptre is the power of a lens with a focal length of 1 meter.
- Sign Convention:
- Convex (converging) lenses have positive (+) power. Used to correct hypermetropia and presbyopia.
- Concave (diverging) lenses have negative (-) power. Used to correct myopia.
- Combinations: For lenses in contact, the net power is the algebraic sum of individual powers:
Additional Aspects
- Binocular Vision: Having two eyes provides:
- A wider field of view.
- Stereopsis (depth perception) because the brain fuses slightly different images from each eye.
- Eye Care:
- Wash eyes regularly with clean water.
- Maintain a proper reading distance (~25 cm).
- Ensure adequate lighting while reading.
- Consume a balanced diet rich in Vitamin A (found in carrots, green vegetables, cod liver oil, eggs, dairy, papaya, mango). Deficiency can cause night blindness.
- Eye Donation:
- A voluntary act that can restore sight to individuals with corneal blindness.
- Eyes can be donated by people of any age or gender.
- Donated eyes must be retrieved within 4-6 hours after death.
The Nature and Behavior of Light
Light is a fundamental form of energy enabling vision. Our understanding highlights its dual nature, behaving as both a wave and a particle depending on the interaction.
Dual Nature of Light
Wave Nature:
- Light exhibits wave-like properties, primarily demonstrated by phenomena like diffraction (bending around small obstacles) and interference (where light added to light can produce darkness, as shown in Young's double-slit experiment).
- Light waves are transverse electromagnetic waves, propagating at a speed
m/s in a vacuum. - Polarisation further confirms the transverse nature of light waves.
- The wave properties are described by frequency (
), wavelength ( ), and velocity ( ), related by . Wavenumber ( ) is also used, defined as .
Particle Nature:
- In interactions with matter (like the photoelectric effect), light behaves as a stream of particles called photons.
- Each photon carries energy
and momentum , where is Planck's constant. - The energy and momentum of a photon depend only on its frequency/wavelength, not the intensity of the light.
- Intensity corresponds to the number of photons per unit area per second.
- This particle nature explains phenomena like the photoelectric effect and radiation pressure.
Quantum Theory:
- Modern quantum theory reconciles these aspects, stating light is neither exclusively a 'wave' nor a 'particle' but exhibits properties of both.
Light Propagation and Interaction with Media
- Speed of Light: The speed of light in a vacuum (
) is constant ( m/s, approximately m/s). Its speed ( ) changes in different media. - Refractive Index (n or μ): A measure of how much light slows down in a medium.
- Absolute Refractive Index:
. - Relative Refractive Index (medium 2 w.r.t. medium 1):
. - If
, the medium slows light (e.g., glass, water).
- Absolute Refractive Index:
- Optical Density: A medium's ability to refract light.
- Higher refractive index (
) means optically denser. - Lower refractive index (
) means optically rarer. - Light travels slower in denser media and faster in rarer media.
- Higher refractive index (
Reflection of Light
Reflection occurs when light bounces off a surface and returns to the same medium.
- Laws of Reflection:
- The incident ray, reflected ray, and the normal to the surface at the point of incidence all lie in the same plane.
- The angle of incidence (
) equals the angle of reflection ( ): .
- Types of Reflection:
- Regular Reflection: Occurs from smooth surfaces (e.g., mirrors), where parallel incident rays remain parallel after reflection.
- Irregular/Diffused Reflection: Occurs from rough surfaces (e.g., walls, paper), where parallel incident rays scatter in various directions.
- Properties during Reflection: Speed, frequency, and wavelength of light remain unchanged.
- Mirrors:
- Plane Mirror: Forms virtual, upright, same-size images.
- Convex Mirror: Forms smaller, virtual images (used in vehicle side mirrors).
- Concave Mirror: Image type (real or virtual) depends on object distance.
- Applications: Mirrors, projectors, periscopes.
Refraction of Light
Refraction is the bending of light as it passes from one medium to another, caused by the change in the speed of light.
- Laws of Refraction (Snell's Law):
- The incident ray, refracted ray, and the normal to the interface at the point of incidence all lie in the same plane.
- The ratio of the sine of the angle of incidence to the sine of the angle of refraction is constant for a given pair of media and color of light:
where and are the absolute refractive indices of medium 1 and medium 2, respectively, and ( ) is the relative refractive index of medium 2 with respect to medium 1.
- Behavior:
- Rarer to Denser: Light slows down, bends towards the normal.
- Denser to Rarer: Light speeds up, bends away from the normal. ( D-R-AW)
- Total Internal Reflection (TIR): Occurs when light travels from a denser to a rarer medium at an angle of incidence greater than the critical angle. The light is completely reflected back into the denser medium. Used in optical fibers and explains the sparkle of diamonds.
Comparison: Reflection vs. Refraction
| Feature | Reflection | Refraction |
|---|---|---|
| Definition | Light bouncing back into the same medium. | Light bending as it enters a different medium. |
| Cause | Light striking a boundary surface. | Change in the speed of light across media. |
| Governing Law | Laws of Reflection ( | Laws of Refraction (Snell's Law: |
| Medium | Occurs in a single medium. | Involves passage between two different media. |
| Speed/λ/ν | Speed, wavelength, frequency unchanged. | Speed and wavelength change, frequency remains unchanged. |
| Example | Image in a mirror. | A pencil appearing bent in water. |
Dispersion of Light
Dispersion is the splitting of white light into its constituent colors (spectrum) when it passes through a refractive medium like a prism.
- Cause: The refractive index (
) of a medium varies slightly with the wavelength ( ) of light. Different colors (wavelengths) bend by different amounts. - Violet light (shorter
) has a higher refractive index and bends most. - Red light (longer
) has a lower refractive index and bends least.
- Violet light (shorter
- Prism Action: Light is refracted twice (upon entry and exit), causing deviation. Since the deviation angle depends on the refractive index, different colors deviate by different amounts, separating them.
- Spectrum (VIBGYOR): The sequence of colors produced.
| Color | Wavelength Range (approx. nm) | Refraction Amount | Refractive Index ( | Deviation |
|---|---|---|---|---|
| Violet | 400 - 450 | Maximum | Highest | Most |
| Indigo | 450 - 475 | High | High | High |
| Blue | 475 - 495 | Medium | Medium | Medium |
| Green | 495 - 570 | Normal | Normal | Normal |
| Yellow | 570 - 590 | Low | Low | Low |
| Orange | 590 - 620 | Very Low | Very Low | Low |
| Red | 620 - 700 | Minimum | Lowest | Least |
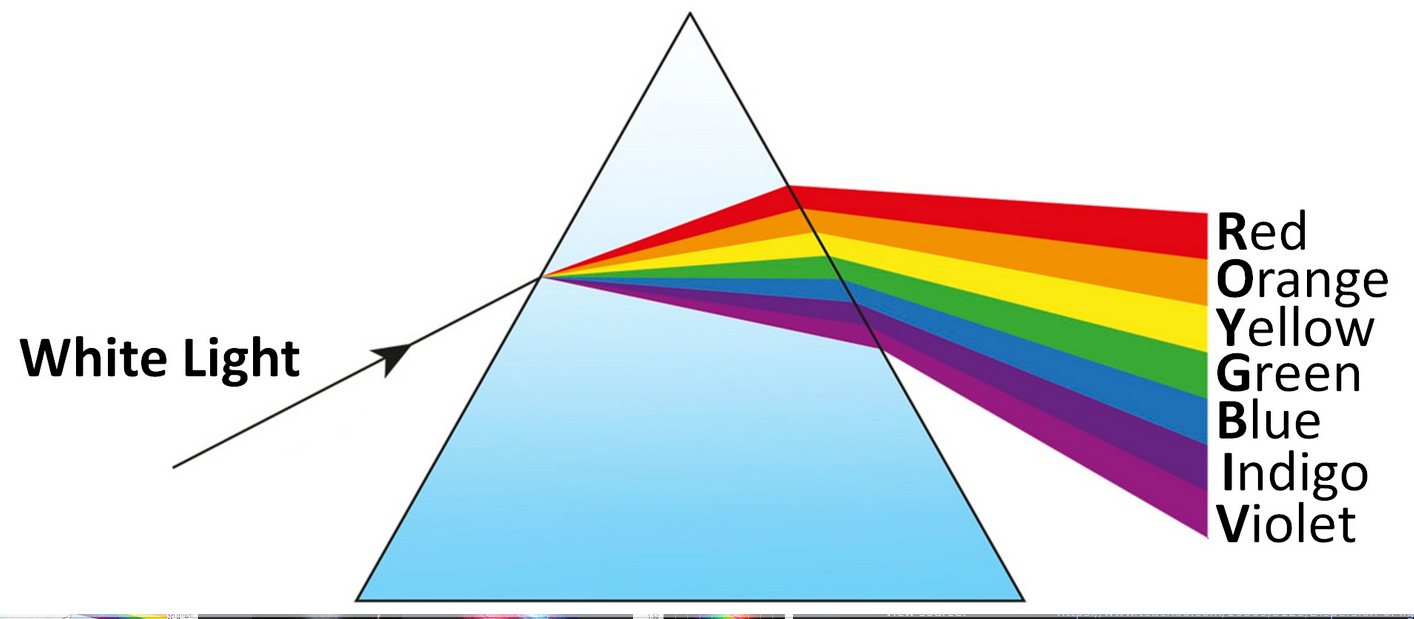
- Newton's Experiment: Demonstrated that white light is composed of these colors by splitting it with one prism and recombining it with an inverted prism.
- Natural Example: Rainbow formation (sunlight dispersed by water droplets).
- Other Effects: Sparkling of diamonds (dispersion combined with TIR), colors in thin oil films (dispersion and interference).
Deviation vs. Dispersion
| Feature | Deviation | Dispersion |
|---|---|---|
| Definition | The bending or change in direction of a light ray. | The splitting of white light into constituent colors. |
| Cause | Change in medium (Refraction). | Wavelength-dependent refractive index. |
| Result | Light ray changes its path. | A spectrum of colors (VIBGYOR) is formed. |
| Example | A single ray bending through a prism. | White light forming a rainbow through a prism. |
Applications of Dispersion
- Spectroscopy: Analyzing substance composition.
- Solar Spectrum Analysis: Studying the Sun's composition.
- Understanding Rainbows.
- Explaining the brilliance of gems like diamonds.
- Designing achromatic lenses to minimize color distortion in optical instruments.
Atmospheric Refraction
Bending of light rays as they pass through Earth's atmosphere due to varying air density and refractive index at different altitudes.
- Effects:
- Twinkling of Stars: Starlight passes through turbulent atmospheric layers with changing refractive indices, causing the apparent position and brightness to fluctuate. Planets, being closer and appearing as extended sources, don't twinkle as the effects average out.
- Advanced Sunrise & Delayed Sunset: Sun appears visible about 2 minutes before actual sunrise and 2 minutes after actual sunset because its light bends around the curve of the Earth due to atmospheric refraction when it's below the horizon.
- Apparent Flattening of Sun/Moon: Near the horizon, light from the bottom edge of the sun/moon travels through denser air and bends more than light from the top edge, making them appear vertically compressed or oval.
Scattering of Light
Scattering occurs when light deviates from its straight path upon striking particles (like dust, water droplets, air molecules).
- Mechanism: Particles absorb light energy and re-emit it in different directions. The nature of scattering depends on the size of the particles relative to the wavelength of light.
- Rayleigh Scattering: Occurs when particles are much smaller than the light's wavelength (e.g., air molecules).
- Scattering intensity (
) is inversely proportional to the fourth power of the wavelength: . - Shorter wavelengths (blue, violet) scatter much more strongly than longer wavelengths (red, orange).
- Scattering intensity (
- Effects of Scattering:
- Blue Color of the Sky: Air molecules scatter blue light from the sun much more effectively than red light across the sky. Our eyes are more sensitive to blue than violet. Without an atmosphere, the sky would be black.
- Reddish Appearance of Sun at Sunrise/Sunset: Sunlight travels through more atmosphere at these times. Most blue light is scattered away, leaving the longer wavelengths (red, orange) to reach our eyes directly.
- White Clouds: Cloud droplets are relatively large compared to light wavelengths. They scatter all colors (wavelengths) roughly equally (Mie scattering), resulting in a white appearance. Denser clouds appear gray or black as they absorb more light.
- Visibility Reduction in Fog/Smoke: Water droplets or smoke particles scatter light, reducing visibility and making headlight beams diffuse.
- Tyndall Effect: The scattering of light by particles in a colloid or a fine suspension, making the beam path visible (e.g., sunbeam in a dusty room, light through fog).
- Raman Effect: Inelastic scattering where the scattered light has a different frequency (and wavelength) than the incident light, providing information about the scattering material's vibrational modes.
Other Concepts Related to Light
- Electromagnetic Spectrum: Light is part of a broad spectrum of electromagnetic waves. Visible light (~400 nm violet to ~700 nm red) is only a small portion.
- Photometry: Measurement of light as perceived by the human eye.
- Luminous Intensity (I): Measured in candela (cd).
- Illuminance (E): Luminous flux per unit area, measured in lux (lx or lm/m²). For a point source:
. - Luminance (L): Brightness of an emitting or reflecting surface.
- Interaction with Matter:
- Absorption/Emission: Atoms/molecules absorb photons to reach excited states and emit photons at specific wavelengths when returning to lower states (creating emission/absorption spectra).
- Photoelectric Effect: Emission of electrons from a material when light shines on it. Requires photon energy (
) to exceed the material's work function ( ). Maximum kinetic energy of ejected electrons: . Demonstrates the particle nature of light. Used in photocells.
- Biological Significance:
- Vision: Human eyes detect visible light, forming images on the retina.
- Photosynthesis: Plants use light energy (especially red and blue) absorbed by chlorophyll to synthesize food.
- Technological Applications:
- Lasers: Produce intense, monochromatic, coherent, and directional light beams.
- Optical Fibers: Transmit light over long distances using total internal reflection.
- Microscopes/Telescopes: Use lenses/mirrors (reflection/refraction) to magnify objects.
Heat, Temperature, and Thermodynamics
Temperature
- Definition: Temperature represents the degree of hotness or coldness of a body. More formally, it's the property determining if a body is in thermal equilibrium with others.
- Heat Flow: Heat spontaneously flows from a body at a higher temperature to one at a lower temperature until thermal equilibrium (equal temperature) is reached.
- Measurement: Measured using a thermometer. Common scales are Celsius (°C), Fahrenheit (°F), and Kelvin (K).
- SI Unit: Kelvin (K). Defined relative to the triple point of water (273.16 K).
is absolute zero - Celsius: Freezing point of water is
, boiling point is . - Fahrenheit: Freezing point of water is
, boiling point is .
- SI Unit: Kelvin (K). Defined relative to the triple point of water (273.16 K).
- Relation to Energy: Temperature is a measure of the average kinetic energy of the molecules within a substance.
Heat
- Definition: Heat (
) is the form of energy transferred between systems due to a temperature difference. It is energy in transit. - Units:
- SI Unit: Joule (J)
- Other Unit: Calorie (cal), where
. (1 calorie raises 1g of water by 1°C from 14.5°C to 15.5°C).
- Nature: Heat is not a state function; a system doesn't "contain" heat. It describes the energy transferred during a process.
- Formula for Temperature Change: The heat required to change the temperature of a substance is given by:
Where: = Heat transferred (J) = Mass of the substance (kg) = Specific Heat Capacity of the substance (J kg⁻¹ K⁻¹ or J kg⁻¹ °C⁻¹) = Change in temperature (K or °C)
Methods of Heat Transfer
Heat is transferred via three primary mechanisms:
- Conduction: Transfer of heat through direct molecular contact within a material (typically solids) without bulk movement of the material itself.
- Example: A metal spoon heating up in hot tea.
- Convection: Transfer of heat by the actual movement of the heated fluid (liquid or gas). Hotter, less dense fluid rises, and cooler, denser fluid sinks.
- Example: Boiling water, sea breezes/land breezes.
- Radiation: Transfer of heat via electromagnetic waves, which requires no medium and can travel through a vacuum.
- Example: Heat from the sun reaching Earth, warmth felt near a fire.
Important Concepts Related to Heat
Specific Heat Capacity (
): The amount of heat energy required to raise the temperature of 1 kg of a substance by 1°C (or 1 K). - Example: Water has a high specific heat (
), making it heat up and cool down slowly, thus moderating climate near large bodies of water. Land has a lower specific heat, heating/cooling faster.
- Example: Water has a high specific heat (
Latent Heat (
): The heat absorbed or released during a change of state (phase transition) without any change in temperature. - Formula:
- Latent Heat of Fusion (
): Heat required to change 1 kg of substance from solid to liquid at its melting point. - Example: Ice melting at
requires .
- Example: Ice melting at
- Latent Heat of Vaporization (
): Heat required to change 1 kg of substance from liquid to gas at its boiling point. - Example: Water turning to steam at
requires . This principle is used in cooling by evaporation (sweating).
- Example: Water turning to steam at
- Formula:
Thermodynamics
Thermodynamics studies heat, temperature, and the conversion of energy between different forms.
Key Terms:
- System: The specific part of the universe being studied.
- Surroundings: Everything outside the system.
- State Variables: Properties defining the system's state (e.g., Pressure
, Volume , Temperature , Internal Energy ). Their values depend only on the current state. Heat ( ) and Work ( ) are not state variables. - Thermal Equilibrium: State where systems in contact have the same temperature and no net heat flows between them.
Laws of Thermodynamics:
- First Law (Conservation of Energy): Energy cannot be created or destroyed, only converted from one form to another. The heat supplied to a system equals the sum of the change in its internal energy and the work done by the system.
Where = heat added, = change in internal energy, = work done by the system. - Second Law (Direction of Processes): Heat naturally flows from a hotter object to a colder object. It also implies that no process can be 100% efficient in converting heat entirely into work in a cycle (some heat must be rejected to a colder reservoir). It introduces the concept of entropy, a measure of disorder, which tends to increase in isolated systems.
- Third Law (Absolute Zero): It is impossible to reach the absolute zero of temperature (
or ) in a finite number of steps.
- First Law (Conservation of Energy): Energy cannot be created or destroyed, only converted from one form to another. The heat supplied to a system equals the sum of the change in its internal energy and the work done by the system.
Temperature Scales and Conversions
| Scale | Symbol | Freezing Point (Water) | Boiling Point (Water) | Absolute Zero | Conversion from Celsius ( |
|---|---|---|---|---|---|
| Celsius | °C | - | |||
| Fahrenheit | °F | ||||
| Kelvin | K | ||||
| Rankine | °R |
Daily Life Examples
| Phenomenon | Concept Illustrated | Explanation |
|---|---|---|
| Spoon handle gets hot in tea | Conduction | Heat travels through the solid spoon material. |
| Boiling water bubbles | Convection | Hotter, less dense water rises. |
| Feeling sun's warmth | Radiation | Heat travels through space (vacuum) via electromagnetic waves. |
| Dark clothes feel hotter | Radiation (Absorption) | Dark surfaces absorb more radiant energy than light surfaces. |
| Cold drink warms up | Second Law | Heat flows from warmer surroundings into the colder drink. |
| Sweating cools the body | Latent Heat of Vaporization | Evaporation of sweat absorbs heat from the skin. |
| Coastal climates are moderate | Specific Heat Capacity (Water) | Water's high specific heat prevents rapid temperature changes. |
| Pressure cooker cooks faster | Effect of Pressure on Boiling Point | Increased pressure raises water's boiling point, allowing higher temperatures. |
| Wearing a blanket feels warm | Insulation (Conduction/Convection) | Trapped air reduces heat loss from the body. |
| Car engine runs | First Law / Heat Engine | Chemical energy (fuel) is converted to thermal energy, then mechanical work. |
Interesting Facts
- Mpemba Effect: Under certain conditions, hot water can freeze faster than cold water.
- Refrigerators and Heat Engines are practical applications governed by the laws of thermodynamics.
- When water freezes, it releases latent heat of fusion, slightly warming the surroundings.
Electrostatics and Current Electricity
Electrostatics
Electrostatics is the study of electric charges at rest, the forces between them, and the electric fields and potentials they create.
Electric Charge
- Charge is a fundamental physical property responsible for electrical phenomena.
- There are two types of charges:
- Positive charge (
): Associated with protons. - Negative charge (
): Associated with electrons.
- Positive charge (
- Fundamental Interaction: Like charges repel, and opposite charges attract.
- Charge is quantized, meaning it exists in discrete units equal to the charge of an electron (
Coulombs). - Charge is conserved; the net charge in an isolated system remains constant.
Coulomb's Law
Coulomb's law quantifies the force (
Where
Examples in Daily Life:
- Hair Standing Up: Combing dry hair transfers charge (comb becomes negative, hair positive), leading to attraction between hair strands and the comb.
- Balloons: Rubbing a balloon gives it a charge (usually negative). It can stick to a neutral wall (by inducing an opposite charge) or repel another similarly rubbed balloon.
- Dust on Screens: TV/computer screens accumulate static charge, attracting dust particles via Coulomb's force.
- Paper Pieces and Scale: A charged scale (rubbed on hair/cloth) induces opposite charges in small paper pieces, causing attraction.
- Clothes Clinging: Friction in a dryer generates static charges on clothes, causing them to stick together.
- Lightning: Charge separation occurs in clouds due to collisions. A large potential difference leads to a massive electrical discharge (lightning) between clouds or to the Earth.
- Plastic Bag and Hand: Rubbing a plastic bag creates static charge, causing attraction to your hand.
- Electrostatic Precipitators: Used in industry to remove dust/pollutants. Particles are charged and then attracted to oppositely charged plates.
- Photocopiers/Laser Printers: Use electrostatic principles to position charged toner particles onto paper.
- Static Shock: Charge builds up on your body (e.g., walking on carpet) and discharges rapidly when touching a conductor (like a metal doorknob).
Electric Field
- An electric field (
) is a vector field existing in the space around a charge or group of charges. It represents the force that would be exerted on a positive test charge ( ) placed at any point in that space. - Definition: The electric field at a point is the electric force (
) experienced by a unit positive test charge ( ) placed at that point: - Important Points:
- If the source charge is positive (
), and are in the same direction for a positive test charge. - If the source charge is negative (
), and are in opposite directions for a positive test charge.
- If the source charge is positive (
- Unit and Dimension:
- SI Unit: Newton per Coulomb (N/C) or Volt per meter (V/m).
- Dimension:
- Characteristics:
- Direction: Points away from positive charges and towards negative charges.
- Strength: Indicated by the magnitude of
. A stronger field exerts a greater force.
- Electric Field Lines: Visual representation of electric fields.
- Originate from positive charges, terminate on negative charges (or extend to infinity).
- Never intersect.
- The density of lines indicates field strength (closer lines mean stronger field).
- The tangent to a field line gives the direction of
at that point. 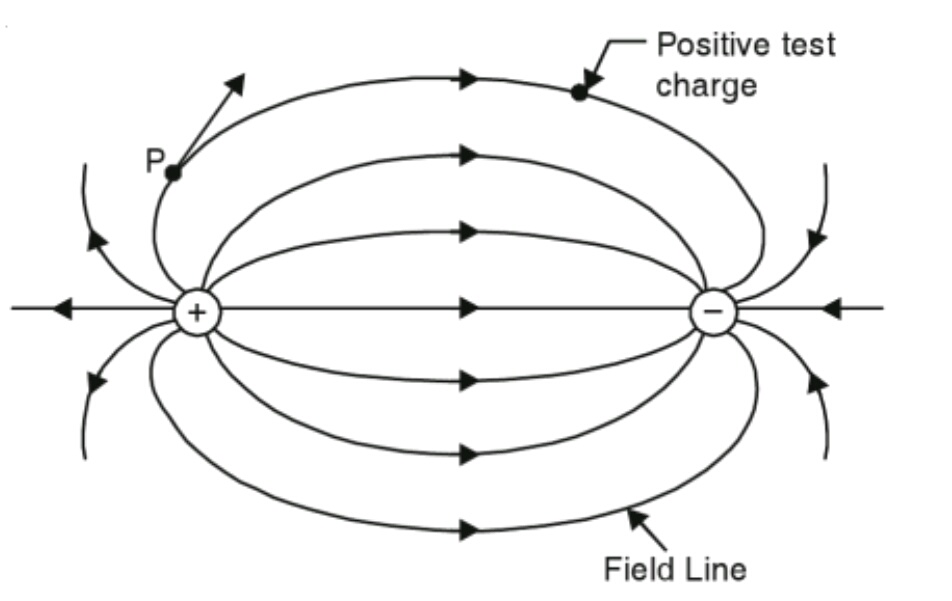
Electric Potential
- Electric potential (
) at a point in an electric field is a scalar quantity representing the electric potential energy ( ) per unit charge ( ) at that point. - Definition: It's defined as the work done (
) by an external force per unit positive charge to move that charge slowly from a reference point (usually infinity) to the point in question: - SI Unit: Volt (V), where
. - Potential due to a Point Charge: The potential (
) at a distance ( ) from a point charge ( ) is: - Relationship with Electric Field: The electric field is the negative gradient of the electric potential. In one dimension:
This means the electric field points in the direction of the steepest decrease in potential. - Key Points:
- Potential is a scalar quantity.
- The potential difference between two points is the work done per unit charge to move a charge between those points.
- All points on the surface of a conductor in electrostatic equilibrium are at the same potential (equipotential surface).
Gauss's Law
- Gauss's Law provides a relation between the electric flux through a closed surface (known as a Gaussian surface) and the net charge enclosed by that surface.
- Definition: The total electric flux (
) through any closed surface is proportional to the total electric charge ( ) enclosed within the surface. - Mathematical Form:
Where represents the surface integral of the electric field over the closed surface. - Simple Explanation: The net number of electric field lines passing outward through a closed surface is proportional to the net positive charge inside. If the net charge is zero, the net flux is zero (equal number of lines enter and leave).
- Key Applications: Useful for calculating electric fields for charge distributions with high symmetry (spherical, cylindrical, planar).
- Field of an infinitely long charged wire.
- Field of a uniformly charged infinite plane sheet.
- Field inside and outside a uniformly charged sphere.
- Key Points:
- A more general formulation related to Coulomb's law.
- Applies to any closed surface.
- Simplifies E-field calculations in symmetric cases.
Capacitor and Capacitance
- A capacitor is a device designed to store electrical energy by storing separated positive and negative charges.
- Typically consists of two conductive plates separated by an insulating material called a dielectric.
- Capacitance (
) is a measure of a capacitor's ability to store charge per unit potential difference. - Definition:
Where is the magnitude of the charge on each plate, and is the potential difference (voltage) between the plates. - Key Points:
- Unit of Capacitance: Farad (F).
. Common units are microfarads ( F) and picofarads (pF). - Capacitance depends on the geometry of the plates (area
, separation ) and the dielectric material ( ) between them. For a parallel plate capacitor: . - Capacitors store energy in the electric field between the plates.
- They block the flow of direct current (DC) once charged but allow alternating current (AC) to pass.
- Unit of Capacitance: Farad (F).
Current Electricity
Current electricity deals with electric charges in motion, constituting an electric current.
Electric Current
- Electric current (
) is the rate of flow of electric charge ( ) through a cross-sectional area of a conductor. - Definition:
Where is the net charge flowing in time . - SI Unit: Ampere (A).
. - Conventional current direction is defined as the direction of flow of positive charge, which is opposite to the direction of electron flow in metallic conductors.
- For current to flow, a closed circuit and a potential difference (voltage source like a battery) are required.
Ohm's Law
- Ohm's Law states that for many materials (called ohmic materials), the current (
) flowing through a conductor is directly proportional to the potential difference ( ) applied across its ends, provided the physical conditions (like temperature) remain constant. - Mathematical Form:
Where is the resistance of the conductor. - SI Unit of Resistance: Ohm (
). .
Resistance and Conductance
- Resistance (
): A measure of a material's opposition to the flow of electric current. - It depends on the material's properties (resistivity,
), its length ( ), and its cross-sectional area ( ): - Resistivity (
) is an intrinsic property of the material (Unit: ). - Resistance generally increases with temperature for conductors.
- It depends on the material's properties (resistivity,
- Conductance (
): The reciprocal of resistance, measuring how easily current flows. - SI Unit: Siemens (S).
.
- SI Unit: Siemens (S).
Factors Affecting Resistance
Based on the formula
- Material: Depends on resistivity (
). Conductors have low , insulators have high . - Length (
): Resistance is directly proportional to length. Longer wires have more resistance. - Cross-sectional Area (
): Resistance is inversely proportional to area. Thicker wires have less resistance. - Temperature: Resistance of most conductors increases with temperature.
Joule's Law of Heating
- When current (
) flows through a resistor ( ) for a time ( ), electrical energy is converted into heat ( ). - Mathematical Form:
This is also equal to . - Unit of Heat Energy: Joule (J).
Kirchhoff's Laws
Used for analyzing complex electrical circuits:
- Kirchhoff's Current Law (KCL) - Junction Rule: The algebraic sum of currents entering any junction (node) in a circuit must equal the algebraic sum of currents leaving that junction.
(Based on conservation of charge) - Kirchhoff's Voltage Law (KVL) - Loop Rule: The algebraic sum of the potential differences (voltages) around any closed loop in a circuit must be zero.
(Based on conservation of energy)
Key Points (Current Electricity Summary)
- Requires a closed path and a potential difference source.
- Current is the flow rate of charge (Amperes).
- Voltage is the potential difference driving the current (Volts).
- Resistance opposes current flow (Ohms).
- Ohm's law (
) relates these quantities for ohmic materials. - Power dissipated is
(Watts). - Energy consumed (often heat) is
(Joules).
Magnetism
Magnetism is a physical phenomenon arising from moving electric charges, producing forces of attraction and repulsion between objects.
Fundamental Concepts
- Magnets produce a magnetic field in the surrounding space.
- Natural magnets (e.g., lodestone) and artificial magnets exist.
- Magnets have two poles: North (N) and South (S). Poles cannot be isolated (no magnetic monopoles).
- Interaction: Like poles repel, unlike poles attract.
Magnetic Field and Field Lines
- The magnetic field (
) is a vector field describing the magnetic influence in a region. - Magnetic field lines are used to visualize magnetic fields:
- Direction: The tangent indicates the direction of
. - Originate from the North pole and terminate on the South pole outside the magnet.
- Form closed loops, continuing inside the magnet from South to North.
- Density of lines indicates field strength (closer lines mean stronger field).
- Never intersect.
- Direction: The tangent indicates the direction of
- SI Unit of Magnetic Field: Tesla (T).
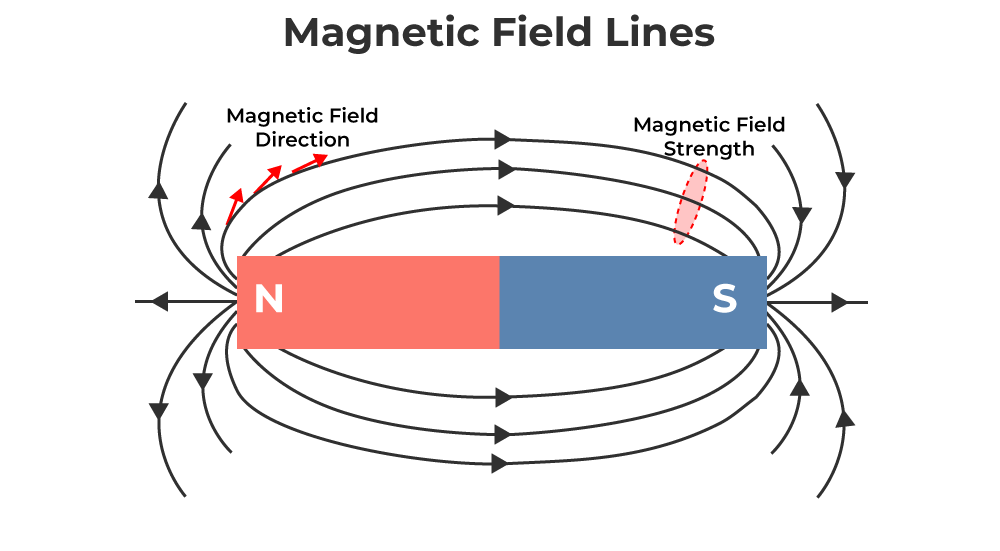
Magnetic Materials
Materials respond differently to magnetic fields:
| Material Type | Behavior in External Field | Magnetic Susceptibility ( | Cause | Examples |
|---|---|---|---|---|
| Diamagnetic | Weakly repelled | Small, negative | Paired electrons, orbital motion | Water, Copper, Bismuth |
| Paramagnetic | Weakly attracted | Small, positive | Unpaired electrons, random atomic moments | Aluminum, Oxygen, Platinum |
| Ferromagnetic | Strongly attracted | Large, positive | Unpaired electrons, aligned magnetic domains | Iron, Nickel, Cobalt |
| Antiferromagnetic | (Complex) | Small, positive | Domains aligned anti-parallel | MnO, Cr₂O₃ |
| Ferrimagnetic | Attracted (like Ferro) | Large, positive | Domains anti-parallel but unequal moments | Ferrites (Fe₃O₄) |
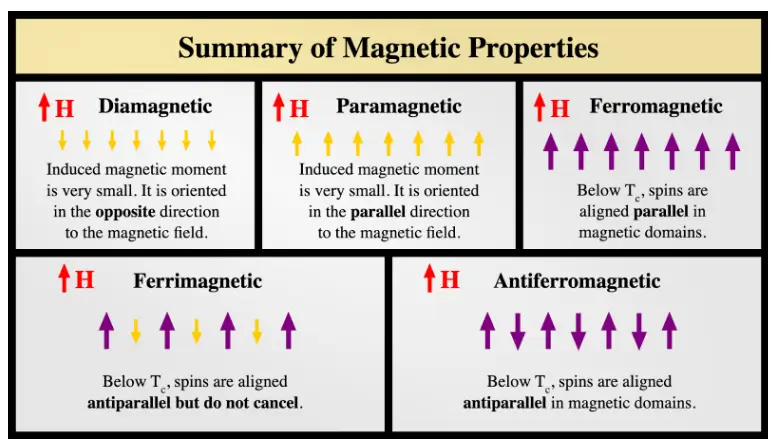 Note: Ferromagnetic materials can be permanently magnetized and lose their property above the Curie temperature.
Note: Ferromagnetic materials can be permanently magnetized and lose their property above the Curie temperature.
Electromagnetism
Electromagnetism is the unified study of electricity and magnetism, recognizing their deep interdependence.
Oersted's Discovery (Magnetic Effect of Current)
- Hans Christian Oersted (1820) discovered that an electric current flowing through a wire creates a magnetic field around it, deflecting a nearby compass needle. This established the fundamental link between electricity and magnetism.
Solenoid and Electromagnet
- A solenoid is a coil of wire. When current flows, it produces a relatively uniform magnetic field inside, similar to a bar magnet.
- An electromagnet is created by inserting a soft iron core into a solenoid. The core enhances the magnetic field significantly. The magnetism can be switched on/off with the current. Used in relays, cranes, bells, MRI machines.
Electromagnetic Induction (Faraday's Law)
- Michael Faraday discovered that a changing magnetic flux through a loop of wire induces an electromotive force (EMF, or voltage) and hence a current in the loop.
- Faraday's Law: The magnitude of the induced EMF (
) is proportional to the rate of change of magnetic flux ( ) through the loop: Where is the number of turns in the coil. is the magnetic flux. - Lenz's Law: The negative sign indicates that the direction of the induced current is such that it creates a magnetic field that opposes the change in magnetic flux that produced it (conservation of energy).
- This principle is the basis for electric generators and transformers.
Force on Moving Charges and Conductors (Lorentz Force)
- A charge (
) moving with velocity ( ) in a magnetic field ( ) experiences a magnetic force ( ): The force is perpendicular to both and . - A current-carrying wire of length (
) carrying current ( ) in a magnetic field ( ) experiences a force: This is the principle behind electric motors. - The total electromagnetic force on a charge
moving in both electric ( ) and magnetic ( ) fields is the Lorentz force:
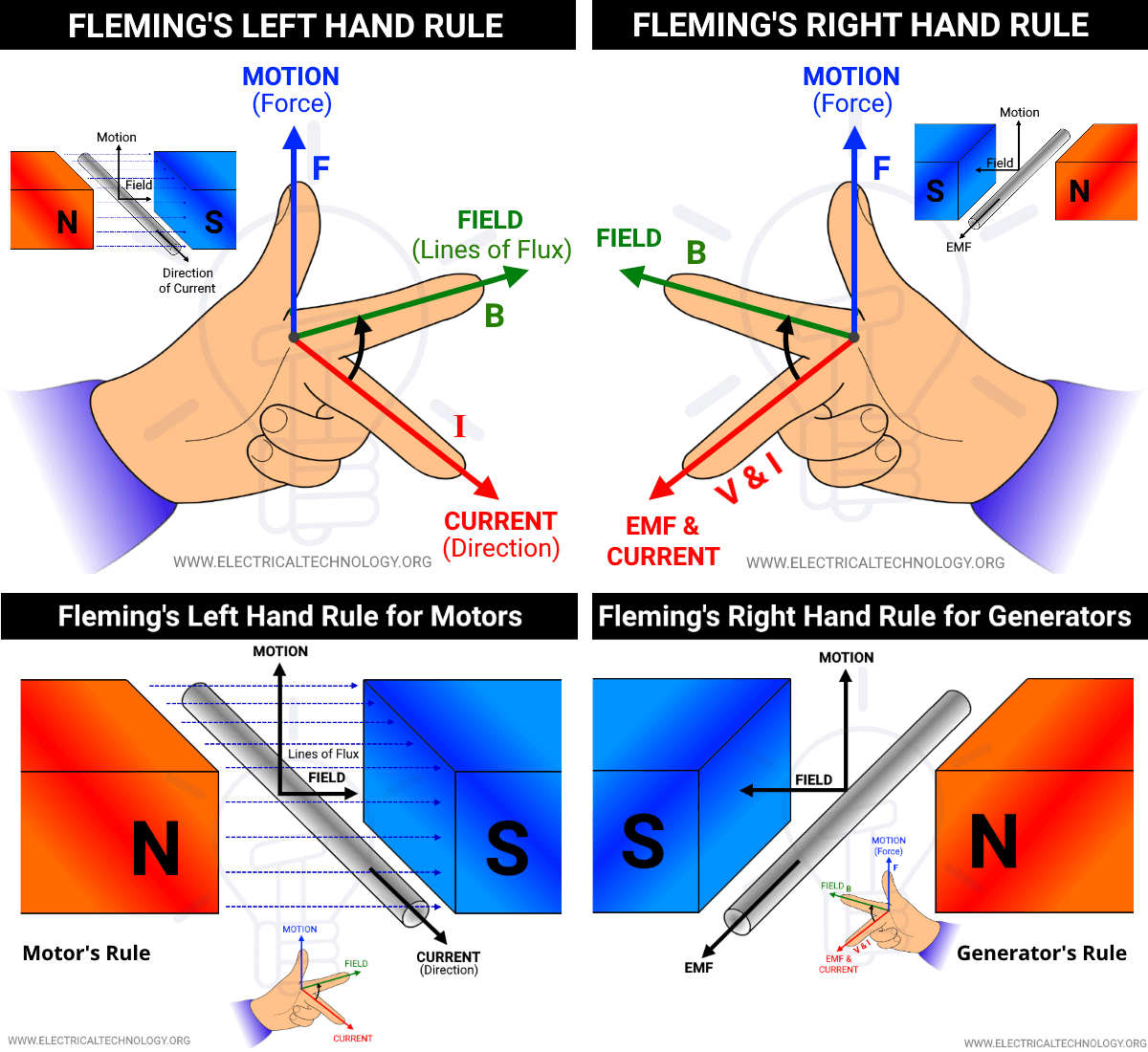
Hand Rules
These mnemonic rules help determine directions in electromagnetism:
| Rule | Purpose | Hand | Fingers/Thumb Representation |
|---|---|---|---|
| Right-Hand Thumb Rule | Direction of Magnetic Field ( | Right | Thumb points in direction of current ( |
| Right-Hand Screw Rule | (Alternative to Thumb Rule) | Right | Direction screw advances is current ( |
| Fleming's Left-Hand Rule | Direction of Force ( | Left | Forefinger = Field ( |
| Fleming's Right-Hand Rule | Direction of Induced Current ( | Right | Forefinger = Field ( |
Applications of Electromagnetism
Electromagnetism underlies vast areas of technology:
- Electric Motors & Generators
- Transformers (stepping voltage up/down)
- Electromagnets (cranes, relays, speakers, MRI)
- Data Storage (magnetic tapes, hard drives)
- Wireless Communication (radio, TV, mobile phones - via electromagnetic waves)
- Medical Imaging (MRI)
- Particle Accelerators (cyclotrons)
Key Distinction: Static vs. Current Electricity
| Feature | Static Electricity | Current Electricity |
|---|---|---|
| Charge State | Charges are accumulated and at rest (or slow) | Charges are in continuous motion (flow) |
| Focus | Charge accumulation, forces, fields, potential | Charge flow rate (current), circuits, resistance |
| Cause | Friction, induction | Potential difference across a conductor |
| Duration | Often transient (e.g., spark discharge) | Can be continuous (as long as circuit is closed) |
| Example | Rubbed balloon sticking to wall, lightning | Electricity powering a light bulb, battery circuit |
Sound
Sound is a mechanical wave produced by vibrations and transmitted through a medium (solid, liquid, or gas). It originates from the vibration of an object, causing compression and rarefaction of the medium's particles. Sound waves are a form of energy that enables us to hear.
Characteristics of Sound Waves
- Frequency (
or ): The number of vibrations or oscillations per second. Its unit is Hertz (Hz). Frequency determines the pitch of a sound (high frequency = high pitch/shrill, low frequency = low pitch/deep). The audible range for humans is approximately 20 Hz to 20,000 Hz. - Time Period (
): The time taken for one complete oscillation. It is the reciprocal of frequency ( ). Its SI unit is second (s). - Wavelength (
): The distance between consecutive compressions or rarefactions. Its unit is meters (m). - Amplitude (
): The magnitude of the maximum disturbance in the medium from its mean position. Amplitude determines the loudness of a sound (larger amplitude = louder sound). - Speed (
): The distance a point on the wave travels per unit time. It depends on the medium's properties (nature, temperature, density) and is related to frequency and wavelength by . Sound travels fastest in solids, slower in liquids, and slowest in gases. - Intensity: The amount of sound energy passing each second through a unit area. Its unit is watts per square meter (W/m²). Often measured in decibels (dB) on a logarithmic scale.
- Loudness: The physiological perception of sound intensity. Related to amplitude and intensity, but subjective. Normal conversation is around 60 dB; sounds above 85 dB can damage hearing.
- Quality/Timbre: Allows distinguishing sounds of the same pitch and loudness from different sources (e.g., violin vs. guitar). It depends on the mixture of the fundamental frequency and overtones (harmonics). A sound of a single frequency is a tone; a mixture is a note.
Production and Propagation
- Sound is produced by vibrating objects (e.g., bell, vocal cords, guitar string).
- Being mechanical waves, sound requires a material medium (solid, liquid, gas) and cannot travel through a vacuum.
- Sound propagates as longitudinal waves in fluids (air, water), where particles oscillate parallel to wave direction, creating compressions (high pressure/density) and rarefactions (low pressure/density).
- In solids, sound can also travel as transverse waves (particles oscillate perpendicular to wave direction) due to shear modulus.
- The disturbance (energy) travels, not the medium's particles themselves over long distances.
Types of Sound Waves (Based on Frequency)
- Audible Sounds: Frequencies between ~20 Hz and 20,000 Hz (human hearing range).
- Infrasonic Sounds (Infrasound): Frequencies below 20 Hz. Inaudible to humans but detectable by some animals (elephants, whales) and produced by earthquakes.
- Ultrasonic Sounds (Ultrasound): Frequencies above 20 kHz. Inaudible to humans but used by animals like bats and dolphins (echolocation) and in various applications.
Speed of Sound
- Depends on the medium's elasticity and density.
- In fluids:
(where is Bulk Modulus, is density). - In solid bars:
(where is Young's Modulus). - In gases:
(Laplace correction, where is the adiabatic index, is pressure).
- In fluids:
- In air at Standard Temperature and Pressure (STP),
m/s. Speed increases with temperature.
Wave Phenomena
- Superposition: When waves overlap, the resultant displacement is the algebraic sum of individual displacements.
- Interference: Superposition of waves with similar frequencies leading to constructive (louder) and destructive (fainter/silent) interference.
- Beats: Periodic variation in loudness when two sounds of slightly different frequencies interfere. Beat frequency
.
- Reflection: Sound waves bounce off surfaces, following the laws of reflection (angle of incidence = angle of reflection).
- Echo: Reflected sound heard distinctly from the original sound. Requires a minimum time delay of ~0.1 s, corresponding to a minimum distance of ~17.2 m to the reflector.
- Reverberation: Persistence of sound due to multiple reflections arriving close together, blending into a continuous sound. Reduced using sound-absorbing materials (foam, curtains).
- Applications of Reflection: Megaphones/horns (directing sound), Stethoscopes (guiding sound), SONAR (underwater ranging), Echolocation (bats, dolphins).
- Doppler Effect: The apparent change in frequency of sound due to relative motion between the source and the observer. Frequency appears higher when approaching, lower when receding. Used in RADAR (though RADAR uses EM waves), astronomy, and medical ultrasound (echocardiography).
Hearing Mechanism
The ear converts sound waves into electrical signals interpreted by the brain:
- Outer Ear (Pinna): Collects sound.
- Auditory Canal: Directs sound to the eardrum.
- Eardrum (Tympanic Membrane): Vibrates.
- Middle Ear (Malleus, Incus, Stapes): Tiny bones amplify vibrations.
- Inner Ear (Cochlea): Fluid-filled structure where vibrations stimulate hair cells.
- Auditory Nerve: Transmits electrical signals from hair cells to the brain.
- The Eustachian tube connects the middle ear to the pharynx, equalizing pressure.
Applications of Sound Waves
- Ultrasonic Waves:
- Ultrasound Imaging: Medical examination of internal organs (e.g., pregnancy scans, echocardiography).
- SONAR (Sound Navigation and Ranging): Measuring ocean depth, locating underwater objects (submarines, shipwrecks).
- Ultrasonic Cleaning: Removing dirt from small parts.
- Gas Leak Detection.
- Other Applications:
- Stethoscope: Listening to heart and lung sounds.
- Microphones & Loudspeakers: Converting sound to electrical signals and vice-versa.
- Sound-based sensors: Security, industrial automation.
- Music and Communication.
Noise Pollution
- Excessive, unwanted sound affecting health and environment.
- Sources: Vehicles, machinery, loudspeakers, aircraft, explosions.
- Effects: Hearing impairment, high blood pressure, stress, sleep disturbance.
- Control: Soundproofing, limiting sources (horns, loudspeakers), planting trees (absorb sound).
Electromagnetic Waves
Electromagnetic (EM) waves are produced by oscillating or accelerated electric charges. They consist of time-varying electric (E) and magnetic (B) fields oscillating perpendicularly to each other and perpendicular to the direction of wave propagation. They are transverse waves and, unlike mechanical waves, do not require a material medium for transmission, allowing them to travel through a vacuum.
Generation
- Maxwell's equations predict that a changing electric field creates a magnetic field, and a changing magnetic field creates an electric field.
- These mutually regenerating fields propagate outward as an EM wave.
- Hertz experimentally confirmed their existence in 1885.
Properties
- Transverse Nature: E and B fields are perpendicular to each other and to the direction of propagation.
- No Medium Required: Can travel through vacuum.
- Speed: Travel at the speed of light (
) in a vacuum ( m/s). The speed is given by , where is the permeability and is the permittivity of free space. In a medium, speed and . - Energy and Momentum: Carry energy and momentum, exert radiation pressure.
- Wave Phenomena: Exhibit reflection, refraction, interference, diffraction.
- Field Relation: The magnitudes of the electric and magnetic fields are related by
. - Spectrum: Encompass a wide range of frequencies and wavelengths.
Electromagnetic Spectrum
EM waves are categorized by frequency (
| Wave Type | Wavelength Range (approx.) | Frequency Range (approx.) | Sources | Applications |
|---|---|---|---|---|
| Gamma Rays | < 0.01 nm | > 30 exahertz (3 × 10¹⁹ Hz) | Radioactive decay, nuclear reactions | Cancer treatment (radiotherapy), sterilization, radioactivity testing |
| X-rays | 0.01 nm - 10 nm | 30 petahertz – 30 exahertz | High-energy electron bombardment, medical tubes | Medical imaging (radiography), industrial inspection, crystal structure analysis |
| Ultraviolet (UV) | 10 nm - 400 nm | 750 terahertz – 30 petahertz | Sun, electric arcs, special lamps | Sterilization, medical treatment (phototherapy), DNA analysis, detecting forgery |
| Visible Light | 400 nm - 700 nm | 430 – 750 terahertz | Sun, lamps, LEDs, lasers, excited atoms | Vision, optical instruments (cameras, telescopes), illumination, photosynthesis |
| Infrared (IR) | 700 nm - 1 mm | 300 gigahertz – 430 terahertz | Hot bodies, molecules, Sun, electronic devices | Heating, thermal imaging (night vision), remote controls, fiber optic communication |
| Microwaves | 1 mm - 1 m | 300 megahertz – 300 gigahertz | Special vacuum tubes (klystrons, magnetrons) | Radar, satellite communication, microwave ovens, wireless networks (Wi-Fi) |
| Radio Waves | > 1 m | < 300 megahertz | Oscillating circuits, antennas | Radio/TV broadcasting, mobile phones, MRI, navigation systems (GPS) |
Maxwell's Equations
Four fundamental equations describing classical electromagnetism:
- Gauss's Law for Electricity: Relates electric field to electric charge distribution (electric field lines originate from positive charges and terminate on negative charges).
- Gauss's Law for Magnetism: States there are no magnetic monopoles (magnetic field lines always form closed loops).
- Faraday's Law of Induction: A changing magnetic flux induces an electromotive force (and hence an electric field). Basis for generators.
- Ampère-Maxwell Law: A magnetic field is created by an electric current or by a changing electric field (Maxwell's displacement current). Basis for electromagnets.
Conclusions: These equations show that electric and magnetic fields are intertwined and that accelerating charges or changing fields generate propagating electromagnetic waves.
Interaction with Matter
EM waves interact with matter via their electric and magnetic fields, causing charges within the material to oscillate. The type of interaction depends on the wave's frequency/wavelength and the material's properties (e.g., microwaves excite water molecules, visible light excites electrons in retina, X-rays penetrate soft tissues).
Daily Phenomena & Applications
- Radio & Mobile Communication: Radio waves transmit signals for radio, TV, mobile phones, Wi-Fi.
- Heating & Remote Controls: Microwaves heat food; Infrared rays are used in heaters and TV remotes.
- Vision & Light: Visible light allows us to see and is used in cameras and lighting.
- Sun Effects: UV rays from the sun cause tanning/sunburn and vitamin D production.
- Medical Imaging: X-rays visualize bones; MRI uses radio waves and magnetic fields for detailed internal images.
- Medical Treatment: Gamma rays treat cancer; UV light sterilizes equipment.
- Technology: Radar (microwaves), night vision (infrared), optical fibers (light/infrared).
Magnetic Resonance Imaging (MRI)
Magnetic Resonance Imaging (MRI) is a medical diagnostic technique employing strong magnetic fields and non-ionising radio waves to generate detailed images of the body's organs, tissues, and physiological or pathological changes. It is particularly effective for imaging soft tissues.
How MRI Works
- Magnetic Field Alignment: A powerful magnet within the MRI machine aligns the protons of hydrogen atoms (abundant in the body's water molecules).
- Radio Wave Excitation: Radio waves are briefly pulsed, knocking these aligned protons out of alignment into a higher energy state.
- Signal Emission & Reception: When the radio waves are turned off, the protons relax back to their aligned state, releasing the absorbed energy as weak radio signals. Sensitive detectors capture these signals.
- Image Creation: Powerful computers process these signals, mapping the distribution and properties of water in different tissues. The varying water content creates contrast, resulting in detailed cross-sectional images.
Characteristics of MRI
- Uses non-ionising radiation, unlike X-rays or CT scans.
- Provides superior contrast and detail for soft tissues (brain, nerves, muscles, ligaments, tendons, organs) compared to techniques like X-rays.
- Weak in imaging hard bony structures, where X-rays excel.
- A painless and non-invasive procedure.
When is MRI Used?
MRI is valuable for diagnosing a wide range of conditions:
- Brain and Spinal Cord: Detecting stroke, tumors, multiple sclerosis, slipped discs, and other neurological issues. Brain structures appear clearer than in X-rays.
- Joints and Bones: Examining arthritis, ligament/tendon injuries, cartilage damage.
- Heart and Blood Vessels: Assessing heart structure and detecting blockages.
- Organs and Tissues: Detecting cancers and diseases in the liver, kidneys, prostate, uterus, etc. It accurately detects pathological and physiological changes.
What Happens During an MRI?
The patient lies on a table that slides into the center of a large, tube-shaped machine containing the magnets. The machine produces loud banging or clicking noises during the scan; earplugs are usually provided. The procedure typically lasts from 15 minutes to over an hour.
Types of MRI
Specialized MRI techniques include:
- Functional MRI (fMRI): Measures brain activity by detecting changes associated with blood flow.
- MR Angiography (MRA): Visualizes blood vessels.
- MR Spectroscopy (MRS): Analyzes biochemical changes in tissues.
- Diffusion Weighted Imaging (DWI): Helps identify stroke and other tissue damage by mapping water molecule diffusion.
Disadvantages of MRI
- Duration: Scans take longer than CT scans.
- Claustrophobia: The confined space can induce anxiety in some patients.
- Cost: Generally more expensive than other imaging methods.
- Metal Restrictions: Cannot be used on patients with certain metallic implants, such as pacemakers, aneurysm clips, or cochlear implants, due to the strong magnetic field.
Nuclear Magnetic Resonance (NMR)
Nuclear Magnetic Resonance (NMR) is a physical and analytical technique leveraging the magnetic properties of certain atomic nuclei to determine the physical and chemical properties of atoms or the molecules containing them. It is fundamental in chemistry, biochemistry, physics, medicine, and pharmaceutical research for understanding atomic-level properties and structures.
Principle of NMR
NMR exploits the phenomenon where specific atomic nuclei (those with a property called spin, like
- Magnetic Field Interaction: When placed in a strong external magnetic field, these nuclei align either with or against the field, creating distinct energy levels.
- Radio Wave Resonance: Applying radio waves of a specific frequency provides energy that causes nuclei in the lower energy state to 'flip' to the higher energy state (resonance).
- Signal Detection: When the radio waves stop, the excited nuclei relax back to their lower energy state, emitting radio waves at a frequency characteristic of the nucleus and its chemical environment. An NMR spectrometer detects these signals.
- Data Analysis: Analyzing the frequencies and patterns of these signals provides detailed information about the structure, dynamics, and composition of molecules.
Applications of NMR
| Field | Applications |
|---|---|
| Chemistry & Pharma | Structure determination (organic/inorganic), drug development/testing, chemical analysis. |
| Biochemistry & Biotech | Protein/nucleic acid structure, enzyme kinetics, biomarker discovery, metabolic studies. |
| Physics & Materials Science | Analysis of solids/polymers/semiconductors, study of atomic/molecular dynamics, material characterization. |
Types of NMR
Common NMR techniques are often named after the nucleus being observed:
H NMR (Proton NMR): Widely used for identifying hydrogen atoms and elucidating structures of organic molecules. C NMR (Carbon-13 NMR): Provides information about the carbon backbone of organic molecules. - Other Nuclei NMR: Techniques like
N, P, Si NMR are used for specific structural studies. - Solid-State NMR: Specialized techniques for analyzing solid materials.
- Dynamic NMR: Studies molecular motion and changes over time.
Advantages of NMR
- Provides detailed molecular structure information non-destructively.
- Allows the study of molecular dynamics and interactions at the atomic level.
- A non-invasive analytical technique.
Limitations of NMR
- Requires nuclei with non-zero spin (e.g.,
H, C, but not C). - Sensitivity can be low, sometimes requiring larger sample amounts or longer acquisition times.
- Requires powerful, expensive superconducting magnets and sophisticated equipment.
- Data analysis often requires specialized expertise.
Difference between NMR and MRI
While both techniques rely on the principles of nuclear magnetic resonance, their applications and focus differ significantly. MRI is essentially a specialized application of the NMR phenomenon tailored for in vivo imaging.
| Feature | NMR | MRI (Magnetic Resonance Imaging) |
|---|---|---|
| Primary Use | Chemical/biological research, materials science | Medical diagnostics |
| Focus | Molecular structure and dynamics at the atomic level | Imaging of tissues and organs based on water distribution |
| Subject | Chemical samples, purified biomolecules, materials | Living organisms (patients) |
| Information Yield | Detailed chemical structure, molecular interactions | Anatomical images, detection of pathological/physiological changes |
| Relationship | Fundamental physical technique | Applied NMR technique using primarily |
Nuclear Fission and Nuclear Fusion
Nuclear fission and nuclear fusion are the two primary methods for generating nuclear energy. Both processes originate within atomic nuclei but differ significantly in their mechanisms, products, energy output, and applications. The energy released in both processes stems from the conversion of mass into energy, governed by Einstein's famous equation
Nuclear Fission
Definition: Nuclear fission is the process where the nucleus of a heavy atom (like uranium, plutonium, or thorium) splits into two or more lighter nuclei, often triggered by the absorption of a low-energy neutron. This splitting releases a substantial amount of energy, along with additional neutrons.
The Fission Process:
- A neutron strikes a heavy, fissile nucleus (e.g.
). - The nucleus absorbs the neutron, becomes highly unstable, and splits into two smaller nuclei (fission fragments, e.g., Barium-141 and Krypton-92).
- This process releases a significant amount of energy (approximately
per fission) primarily as kinetic energy of the fragments and gamma rays. - Typically, 2-3 additional neutrons are also released.
Chain Reaction: The neutrons released during fission can go on to induce fission in other nearby heavy nuclei. This creates the possibility of a self-sustaining chain reaction.
- In nuclear reactors, this chain reaction is carefully controlled using moderators (to slow neutrons) and control rods (to absorb excess neutrons), releasing energy at a steady rate.
- In nuclear weapons, the chain reaction is allowed to proceed uncontrolled, leading to a rapid, explosive release of energy.
Fissile Materials: Materials capable of sustaining a fission chain reaction with thermal (slow) neutrons are called fissile. Key examples include
Characteristics of Nuclear Fission:
| Feature | Description |
|---|---|
| Process | Splitting of a heavy nucleus |
| Fuel | Heavy elements (Uranium, Plutonium, Thorium) |
| Trigger | Neutron bombardment |
| Energy Output | High (~ |
| Neutron Output | 2-3 neutrons per fission, enabling chain reactions |
| Waste | Significant radioactive waste with long half-lives |
| Control | Can be controlled (reactors) or uncontrolled (weapons) |
| Occurrence | Utilized in reactors/weapons; rare natural occurrences |
Applications of Nuclear Fission:
- Nuclear Power Plants: Controlled fission generates heat to produce steam, driving turbines for electricity generation. This is a major source of low-carbon electricity worldwide. Nuclear power reactors in India are located at Tarapur, Rana Pratap Sagar, Kalpakkam, Narora, Kakrapar, and Kaiga.
- Nuclear Weapons (Atomic Bombs): Uncontrolled fission chain reactions create massive explosions (e.g., Hiroshima, Nagasaki).
- Nuclear Propulsion: Compact nuclear reactors power submarines and aircraft carriers, allowing long operation without refueling (e.g., USS Enterprise, Typhoon-class submarines).
- Medical Applications (Nuclear Medicine): Fission produces radioisotopes used in:
- Cancer Therapy (Radiotherapy): E.g., Cobalt-60 (
). - Medical Imaging: E.g., Technetium-99m (
) for scans. - Treatment: E.g., Iodine-131 (
) for thyroid disorders.
- Cancer Therapy (Radiotherapy): E.g., Cobalt-60 (
- Industrial Applications:
- Material Analysis/Inspection: Neutron radiography.
- Food Preservation: Irradiation to extend shelf life.
- Space Exploration: Radioisotope Thermoelectric Generators (RTGs), often using
produced in reactors, provide power for deep space missions (e.g., Voyager, Curiosity, Perseverance). - Research and Education: Reactors provide neutron sources for physics, chemistry, materials science, and biological research.
- Seawater Desalination: Heat from reactors can be used to desalinate water.
Hazards of Nuclear Fission: The primary hazard is the production of radioactive waste, which remains dangerous for thousands of years and requires careful long-term management. Accidental leakage of radioactive materials from power plants poses serious health and environmental risks, as radiation can cause mutations, cancer, and other disorders in biological organisms.
Nuclear Fusion
Definition: Nuclear fusion is the process where two light atomic nuclei combine, or fuse, under conditions of extremely high temperature and pressure to form a single, heavier nucleus, releasing a vast amount of energy.
The Fusion Process: A common reaction involves hydrogen isotopes:
- Deuterium (
or D) and Tritium ( or T) nuclei collide at immense speeds due to temperatures exceeding 10 million degrees Celsius ( or ). - They overcome their mutual electrostatic repulsion and fuse.
- The fusion produces a Helium-4 nucleus (
) and a high-energy neutron. - This process releases significant energy (~
per D-T reaction).
Conditions Required: Fusion requires overcoming the strong electrostatic repulsion between positively charged nuclei. This necessitates:
- Extremely High Temperatures: Millions of degrees Celsius/Kelvin to give nuclei enough kinetic energy. At these temperatures, matter exists as plasma (a hot, ionized gas).
- High Pressure/Density: To increase the probability of collisions. Confining this high-temperature plasma is a major technological challenge, often approached using strong magnetic fields (e.g., in Tokamak devices like ITER).
Energy Source of Stars: Nuclear fusion is the fundamental energy source of stars, including our Sun. In stellar cores, hydrogen nuclei fuse to form helium under immense gravitational pressure and temperature, releasing the energy that makes stars shine.
Characteristics of Nuclear Fusion:
| Feature | Description |
|---|---|
| Process | Joining (fusion) of light nuclei |
| Fuel | Light elements (Hydrogen isotopes: Deuterium |
| Conditions | Extremely high temperature ( |
| Energy Output | Very high (~ |
| Neutron Output | Typically 1 neutron per D-T fusion reaction |
| Waste | Little long-lived radioactive waste; Helium is inert |
| Control | Sustained controlled fusion not yet achieved commercially |
| Occurrence | Powers stars (Sun); used in hydrogen bombs; experimental reactors |
Potential Applications and Advantages:
- Clean Energy Production: Fusion holds the potential for a nearly limitless, safe, and environmentally friendly energy source.
- Abundant Fuel: Deuterium is readily available in seawater. Tritium can be bred from Lithium.
- Safety: Fusion reactions are not inherently chain reactions and are easier to stop; less risk of meltdown.
- Minimal Waste: Produces mainly helium, with some shorter-lived radioactive components in reactor structures, but far less long-lived waste than fission. Experimental reactors like ITER aim to demonstrate feasibility.
- Hydrogen Bomb (Thermonuclear Weapons): Uncontrolled fusion, triggered by a fission bomb, releases enormous energy.
- Space Exploration & Propulsion: Fusion rockets could offer higher thrust and efficiency for faster interplanetary and interstellar travel.
- Industrial & Scientific Research: High-temperature plasmas have applications in materials science and fundamental physics research.
- Medical Applications: Neutrons from fusion could potentially be used for neutron therapy or isotope production.
Challenges of Nuclear Fusion: The primary challenge is achieving ignition – creating and sustaining conditions (temperature, pressure, confinement time) where the fusion reaction produces more energy than is required to initiate and maintain it. Confining the incredibly hot plasma is extremely difficult.
Comparison: Nuclear Fission vs. Nuclear Fusion
| Feature | Nuclear Fission | Nuclear Fusion |
|---|---|---|
| Definition | Splitting a heavy nucleus into lighter ones | Combining light nuclei into a heavier one |
| Fuel | Heavy elements (Uranium | Light elements (Hydrogen isotopes |
| Conditions | Neutron bombardment; critical mass | Extremely high temperature ( |
| Energy Release | High (~ | Very high (~ |
| Chain Reaction | Yes (can be controlled or uncontrolled) | No inherent chain reaction in the same way |
| Waste Products | Significant long-lived radioactive waste | Little long-lived radioactive waste; Helium product |
| Natural Example | Rare natural reactors (Oklo); induced processes | Sun and stars |
| Current Technology | Established (Power plants, weapons) | Experimental (ITER); Hydrogen bombs |
| Control | Technology well-developed | Achieving sustained control is a major challenge |
| Safety Concerns | Radioactive waste, meltdown risk, proliferation | Plasma control challenges, tritium handling, neutron activation |
Quick Revision
Gravitation
- Definition: Attractive force between any two objects with mass. Universal.
- Newton's Law:
. (F=force, m=mass, r=dist betw centers, G=Universal Grav. Const ) - Characteristics: Attractive only, Universal,
, (Inverse Square), Acts along line, Weakest fundamental force but infinite range (dominant on cosmic scale). - Acc. Due to Gravity (g): Free fall acc. on a planet.
. Independent of falling object's mass. Avg Earth . - Variation in g:
- Altitude: Decreases w/ height (
). - Depth: Decreases w/ depth (
), at center. - Latitude: Weaker at equator (bulge/rotation).
- Altitude: Decreases w/ height (
- Kepler's Laws (Planetary Motion):
- Orbits: Elliptical, Sun at one focus.
- Areas: Equal areas swept in equal time (planets faster near Sun). Cons. of Angular Momentum.
- Periods:
(a=semi-major axis). .
- Escape Velocity (Ve): Min speed to escape grav. pull.
. Earth . Higher M or smaller R = Higher . Black Hole: . - Satellite Motion: Gravity provides centripetal force for orbit.
- Orbital Velocity:
( ). - Period:
. Higher orbit longer period. - Types: Geostationary (GEO): Equator, $\approx$36k km, 24h, fixed view. Polar (LEO): Low alt, short period, covers poles/entire Earth over time.
- Orbital Velocity:
- Gravitational Potential Energy (U):
- Near surface:
. - General:
(Zero at ). Always negative.
- Near surface:
- Daily Life: Keeps us grounded (Weight
), falling objects, tides (Moon/Sun grav), orbits, fluid flow (rivers), climbing.
The Human Eye and Vision
- Function: Camera-like organ, captures light, converts to brain signals.
- Structure:
- Outer: Sclera (white), Cornea (transparent front, refracts most light, no vessels).
- Middle (Uvea): Choroid (vessels), Ciliary Body/Muscles (control lens shape), Iris (colored, controls pupil size), Pupil (opening, regulates light).
- Inner: Retina (light-sensitive, back layer). Rods (dim light), Cones (color/bright light). Macula/Fovea (sharpest vision). Optic Nerve (signals to brain). Blind Spot (no photoreceptors).
- Internal: Lens (transparent, biconvex, flexible, fine focus), Aqueous Humor (fluid betw cornea-lens, nourishes), Vitreous Humor (jelly behind lens, shape/support).
- Mechanism: Light -> Cornea/Aqueous/Lens (refract) -> Pupil (adjusts) -> Retina (inverted real image) -> Rods/Cones (light to signals) -> Optic Nerve -> Brain (interprets upright).
- Accommodation: Lens changes focal length to focus. Muscles relaxed = Lens flatter (long f, distant). Muscles contracted = Lens thicker (short f, near).
- Near Point: Closest clear vision (
). - Far Point: Farthest clear vision (
for normal eye).
- Near Point: Closest clear vision (
- Vision Defects:
- Myopia (Nearsighted): Distant blurry. Eyeball too long/Lens too powerful. Corrected by Concave lens.
- Hypermetropia (Farsighted): Near blurry. Eyeball too short/Lens too weak. Corrected by Convex lens.
- Presbyopia (Age): Near blurry. Stiff lens/Weak muscles. Corrected by Convex (Bifocal).
- Astigmatism: Blurred/distorted (all dist). Irregular cornea/lens. Corrected by Cylindrical lens.
- Cataract: Cloudy lens. Surgery.
- Power of Lens (P):
(f in meters). Unit: Dioptre (D). Convex +P, Concave -P. . - Care: Vitamin A vital (prevents night blindness). Maintain distance/light. Eye donation possible (cornea).
The Nature and Behavior of Light
- Dual Nature: Behaves as both wave (Diffraction, Interference, Polarization) and particle (Photons: Photoelectric Effect, Radiation Pressure). Quantum theory unifies.
- Propagation: Transverse EM wave.
in vacuum. . Speed changes in medium ( ). - Refractive Index (n):
. Higher n = Slower light = Optically denser. - Reflection: Bounces off surface (same medium).
. Speed, , UNCHANGED. Mirrors. - Refraction: Bends passing into another medium. Cause: Change in speed. Snell's Law:
. Rarer to Denser: Bends Towards Normal. Denser to Rarer: Bends Away from Normal. - Total Internal Reflection (TIR): Denser to Rarer,
critical angle. Light reflected back. Optical fibers, diamonds. - Dispersion: Splitting white light into colors (VIBGYOR) by prism. Cause:
varies with . Violet (short ) bends most (high n), Red (long ) bends least (low n). Rainbows. - Atmospheric Refraction: Bending in atmosphere. Effects: Twinkling stars, Advanced sunrise/Delayed sunset (~2min), Flattened sun/moon near horizon.
- Scattering: Deviation by particles.
- Rayleigh: Particles <<
. . Blue sky (blue scatters most), Red sun (blue scattered away). - Mie: Particles
. White clouds (all scatter equally). - Tyndall effect.
- Rayleigh: Particles <<
- EM Spectrum: Gamma, X-rays, UV, Visible, IR, Microwaves, Radio (decreasing
, increasing ). Visible: . - Photoelectric Effect: Light releases electrons.
. Proves particle nature. - Applications: Vision, lighting, communication (radio, TV, mobile), heating (IR, microwave), medical (X-ray, UV, therapy), technology (lasers, optical fibers, radar).
Heat, Temperature, and Thermodynamics
- Temperature (T): Degree of hotness/coldness. Average KE of molecules. SI Unit: Kelvin (K).
Absolute Zero. - Heat (Q): Energy transferred due to
. Energy in transit. SI Unit: Joule (J). . . Not a state function. - Heat Transfer:
- Conduction: Direct contact (solids).
- Convection: Fluid movement (liquids/gases).
- Radiation: EM waves (no medium).
- Specific Heat Capacity (c): Heat to raise 1kg by 1°C/1K. High c means heats/cools slowly (water).
- Latent Heat (L): Heat for phase change at constant T.
. Fusion ( , solid-liquid), Vaporization ( , liquid-gas). Evaporation cools (uses ). - Thermodynamics: Study of heat, T, energy conversion. System, Surroundings, State Variables (p, V, T, U).
- Laws:
- 1st Law (Energy Cons.):
. - 2nd Law (Direction): Heat flows Hot to Cold. Entropy increases. Efficiency limit for engines.
- 3rd Law (Absolute Zero): Unreachable.
- 1st Law (Energy Cons.):
- Temp Scales: Celsius (°C), Fahrenheit (°F), Kelvin (K). Conversions:
, . . - Examples: Spoon in tea (Conduction), Boiling (Convection), Sun (Radiation), Sweating (Latent Heat), Coastal climate (Specific Heat).
Electrostatics and Current Electricity
- Electrostatics: Charges at rest.
- Charge: + (proton), - (electron). Like repel, opposite attract. Quantized (
), Conserved. - Coulomb's Law:
. Force betw point charges. - Electric Field (
): Force per unit + charge. . Unit N/C or V/m. Lines out of +, into -. Density Strength. - Electric Potential (V): Energy per unit charge.
. Unit Volt (V=J/C). Scalar. . Conductor surface is equipotential. - Gauss's Law:
. Flux enclosed charge. Useful for symmetric E-field calculations. - Capacitor: Stores charge/energy.
. Unit Farad (F). & dielectric ( ). Blocks DC, passes AC. - Current Electricity: Charges in motion.
- Current (I): Rate of charge flow.
. Unit Ampere (A=C/s). Conventional current (+ve flow). Needs closed circuit & potential difference (V). - Ohm's Law:
. Relation betw V, I, R for ohmic materials (const T). - Resistance (R): Opposes current.
. Depends on material ( ), length (l), area (A), temperature. Unit Ohm ( ). Conductance (Siemens). - Joule's Law of Heating: Heat
. Energy converted to heat. - Kirchhoff's Laws: KCL (Junction Rule):
(Charge Cons.). KVL (Loop Rule): (Energy Cons.). - Magnetism: Moving charges produce magnetic fields. N/S poles (no monopoles). Like repel, unlike attract.
- Magnetic Field (
): Unit Tesla (T). Lines: N to S (outside), S to N (inside), closed loops. - Electromagnetism: Unified E & M.
- Oersted: Current
B field. - Faraday: Changing
induced EMF/Current. . Lenz's Law opposes change. Generators. - Lorentz Force: Force on charge/current in B field.
or . Motors.
- Oersted: Current
- Hand Rules: RHR (Current
B), Fleming's LHR (Force on current), Fleming's RHR (Induced current). - Applications: Motors, Generators, Transformers, Electromagnets, Communication (EM waves), MRI.
Sound
- Definition: Mechanical wave from vibrations, needs a medium (solid, liquid, gas - NO vacuum). Propagates as longitudinal waves in fluids (C/R). Energy travels.
- Characteristics:
- Frequency (
): Pitch (Hz). Audible: 20Hz-20kHz. Infrasound (<20Hz), Ultrasound (>20kHz). - Time Period (T):
(s). - Wavelength (
): Distance C-C / R-R (m). - Amplitude (A): Loudness.
- Speed (
): Fastest in solids > liquids > gases. Depends on elasticity/density. . - Intensity (W/m²): Energy flow rate. Measured in Decibels (dB).
- Quality/Timbre: Distinguishes sources (harmonics).
- Frequency (
- Wave Phenomena:
- Superposition: Interference (Constructive/Destructive). Beats (
). - Reflection: Bounces off surfaces. Echo (distinct reflection, >0.1s delay, >17.2m dist). Reverberation (multiple reflections, blending). Applications: SONAR, Echolocation, Stethoscope.
- Doppler Effect: Apparent
change due to source/observer motion (approaching , receding ).
- Superposition: Interference (Constructive/Destructive). Beats (
- Hearing: Ear converts sound waves to electrical signals. Outer
Eardrum (vibrate) Middle bones (amplify) Inner Cochlea (fluid/hair cells) Auditory nerve Brain. - Applications: Ultrasound (Medical imaging, SONAR, Cleaning), Stethoscope, Music/Communication.
- Noise Pollution: Harmful unwanted sound. Control: Soundproofing, limits.
Electromagnetic Waves (EM Waves)
- Definition: Oscillating E and B fields
to each other and to propagation. Produced by accelerating charges. Transverse waves. NO medium needed (travel in vacuum at ). - Generation: Maxwell's equations (Changing E creates B, Changing B creates E).
- Properties: Transverse, No medium, Speed
in vacuum ( in medium), Carry Energy/Momentum, Wave phenomena (reflect, refract, etc.), . - EM Spectrum (Increasing
): - Gamma Rays (<0.01nm): Nuclear. Radiotherapy, Sterilization.
- X-rays (0.01-10nm): Electron bombardment. Medical imaging, Inspection.
- UV (10-400nm): Sun, lamps. Sterilization, Phototherapy.
- Visible Light (400-700nm): Sun, lamps. Vision, Optics, Photosynthesis.
- Infrared (IR) (700nm-1mm): Hot bodies, Sun. Heating, Thermal imaging, Remotes.
- Microwaves (1mm-1m): Special tubes. Radar, Sat comm, Ovens, Wi-Fi.
- Radio Waves (>1m): Oscillating circuits. Broadcasting, Mobile phones, MRI.
- Interaction: Fields interact with charges in matter. Depends on wave
/ and material. - Applications: Pervasive in technology (Comm, Heating, Medical, Imaging, etc.).
Magnetic Resonance Imaging (MRI)
- Definition: Medical imaging using strong magnetic fields + non-ionising radio waves for detailed soft tissue images.
- How it Works: B field aligns H+ protons
Radio pulse excites protons Protons relax, emit radio signals Computer processes signals (water distribution/properties) Image. - Characteristics: Non-ionising, Superior soft tissue contrast, Weak on bone, Painless, Non-invasive. Detects pathological/physiological changes.
- Uses: Brain/Spinal cord, Joints, Organs, Heart.
- Disadvantages: Long scan, Claustrophobia, Costly, Cannot use with certain metal implants (pacemakers, clips).
Nuclear Magnetic Resonance (NMR)
- Definition: Analytical technique using magnetic properties of nuclei (
H, C) to determine molecular structure/properties. - Principle: Nuclei with spin align in B field
Radio wave 'flips' nuclei (resonance) Nuclei relax, emit characteristic signals Analyze signals for structure/dynamics. - Applications: Chemical analysis, Drug structure, Protein structure, Materials science.
- Advantages: Detailed molecular structure (non-destructive), Studies dynamics, Non-invasive (sample).
- Limitations: Requires specific nuclei, Can be low sensitivity, Expensive equipment (superconducting magnets), Requires expertise.
- NMR vs MRI: NMR = fundamental technique, molecular structure (samples). MRI = applied NMR, medical imaging (patients, water distribution).
Nuclear Fission and Nuclear Fusion
Both: Release huge energy from mass-energy conversion (
). Energy in MeV scale. Nuclear Fission:
- Process: Heavy nucleus (U, Pu) splits (often by neutron).
- Fuel: Heavy elements (U-235, Pu-239).
- Trigger: Neutron bombardment.
- Energy: High (~200 MeV/fission).
- Output: 2-3 neutrons + Fission fragments + Energy.
- Chain Reaction: Yes (controlled in reactors, uncontrolled in weapons).
- Waste: Significant long-lived radioactive waste.
- Occurrence: Reactors, Weapons, Rare Natural.
- Applications: Nuclear Power (locations in India: Tarapur, etc.), Weapons, Propulsion, Medical (radioisotopes), Industry, Space RTGs.
- Hazards: Radioactive waste, Accidental leakage (radiation risks).
Nuclear Fusion:
- Process: Two light nuclei (D, T) combine/fuse.
- Fuel: Light elements (H isotopes: Deuterium, Tritium).
- Conditions: Extremely High Temperature (
K, plasma) and Pressure. - Energy: Very High (~17.6 MeV/D-T), higher per unit mass than fission.
- Output: He nucleus + Neutron + Vast energy.
- Chain Reaction: No inherent chain reaction.
- Waste: Little long-lived radioactive waste (He is inert).
- Occurrence: Powers Sun/Stars, Hydrogen bombs, Experimental reactors (ITER).
- Potential: Clean, near-limitless energy source (abundant fuel, safer, minimal waste).
- Challenges: Achieving/Sustaining ignition (energy gain), Plasma confinement (e.g., Tokamak).
- Applications: H-bombs, Potential for clean power, Space propulsion.